
SOLUTIONS > PROCESS
Audit Management
Be audit-ready at all times

Streamline audit management
In the life sciences industry, a well-executed audit management process supports compliance, ensures product quality, and reduces risks.

Automate processes
Automated processes save time, reduce errors, and are easier to manage.
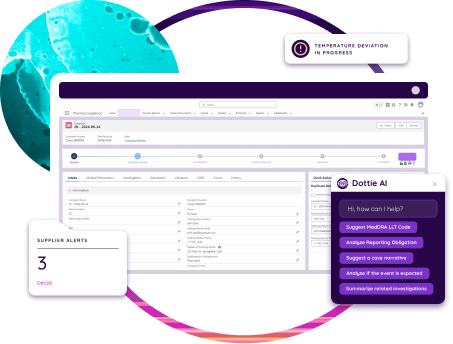
Unify data
Integrated systems enable efficient audit planning, execution, and reporting.

Enhance visibility
Easily track audit progress and identify potential issues in real-time.

Dot Compliance’s powerful out-of-the-box audit management software solution allows life science organizations to cost effectively manage their end-to-end audit life cycle quickly and precisely, simplifying the entire audit process. Prepare for and conduct audits and ensure that internal/external audits are being conducted correctly.
Why Dot Compliance Audit Management
Eliminate paper trails with an “audit ready” anytime, anywhere electronic solution
Dot Compliance’s ready-to-use eQMS solutions help life sciences manufacturers simplify and organize the entire audit management process.
Hassle-free audit process
Step-by-step approval and review process with electronic signatures and approval matrix.
Single workflow for complete visibility
With Dot Compliance, organizations can track, maintain, and improve processes related to applicable audits and regulations.
Streamline Supplier Audits
Built-in audit management capabilities that extend out to suppliers allow the organization to automate workflow, data, and records related to supplier audits.

The eQMS that keeps you one step ahead
Dot Compliance is the industry’s first AI-powered eQMS, built to get you up and running fast – and to give you the insights to drive quality efficiently.
Smart solutions for life sciences companies at every stage
Meet the eQMS that evolves with you, from setting a foundation with core processes to the industry’s most sophisticated AI quality and compliance intelligence.



